
The 2015-2016 El Niño will go down as one of the strongest on record, and also, thanks to El Niño Rapid Response Campaign, one of the best observed.
ENSO is a complicated thing to model. What are the challenges, and how can we overcome them?
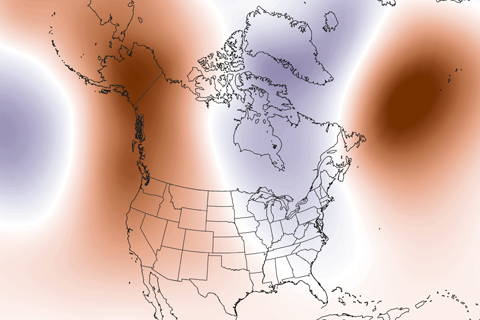
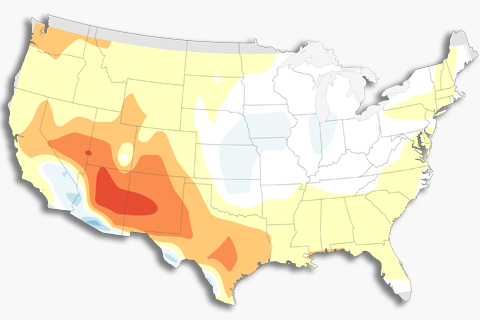
Guest blogger Dennis Hartmann makes the case that warm waters in the western tropical Pacific—part of the North Pacific Mode climate pattern—are behind the weird U.S. winter weather of the past two seasons.
How do we verify forecasts that use probabilities? Read on to find out.
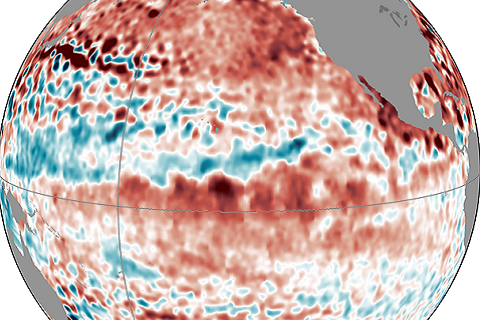
Forecasters are still calling for a 65% chance of El Nino conditions being met in the next few months. Isn't this late for the start of an ENSO event?
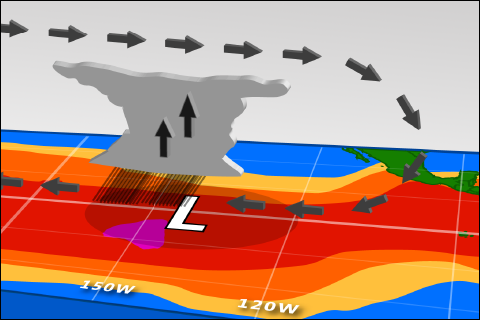
Sea surface temperatures are up. So why haven't forecasters declared El Niño conditions?
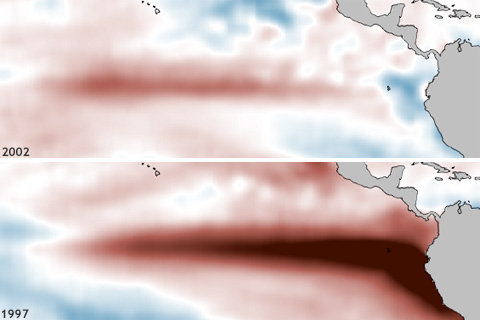
One of ENSO’s most important influences is to the Indian Monsoon—the large-scale circulation pattern that brings the Indian subcontinent the vast majority of its yearly rainfall. And while La Niñas tend to increase monsoon rainfall, the monsoon’s relationship with El Niño can be a little more complicated.
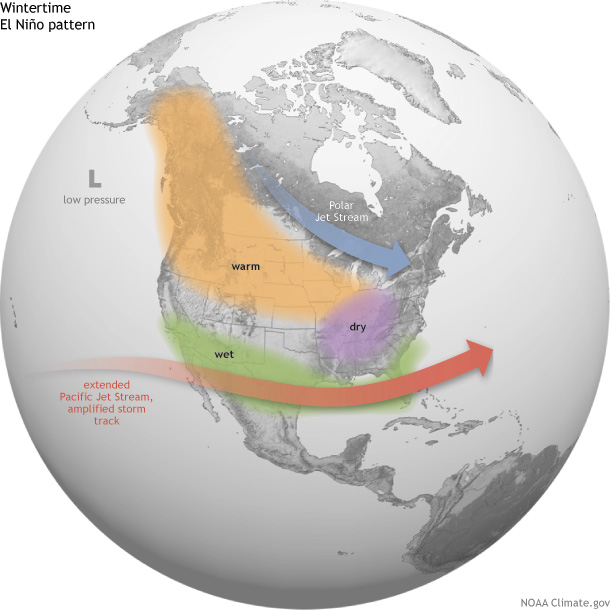
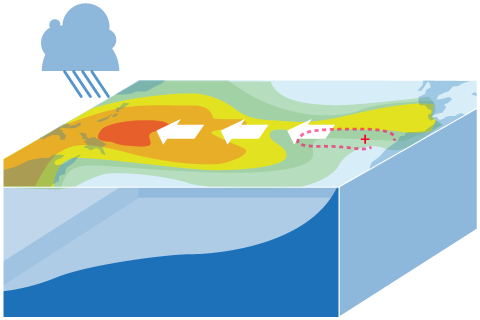
How does El Niño affect U.S. winter temperature and precipitation?