The January 2023 climate outlook favors a wetter-than-average start to the new year for the western US, northern Plains, Great Lakes and Tennessee Valley, and a warmer-than-average month for the central and eastern United States.
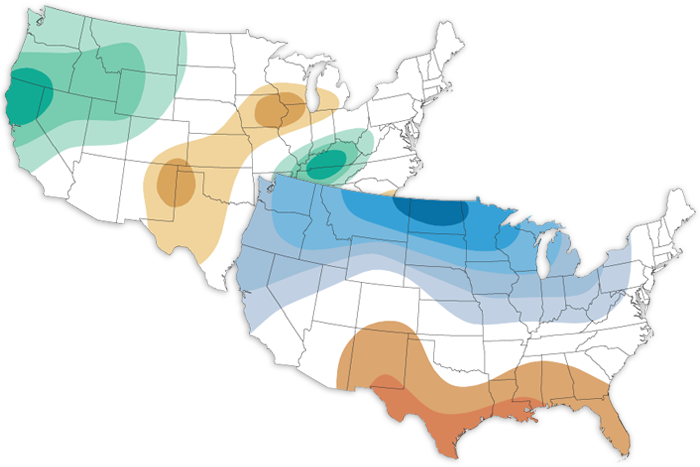
The December 2022 climate outlook favors a colder-than-average month across the northern US, and a warmer-than-average month across the southern US. Meanwhile, odds are tilted towards a wetter-than-average December for the West and Ohio and Tennessee Valleys.
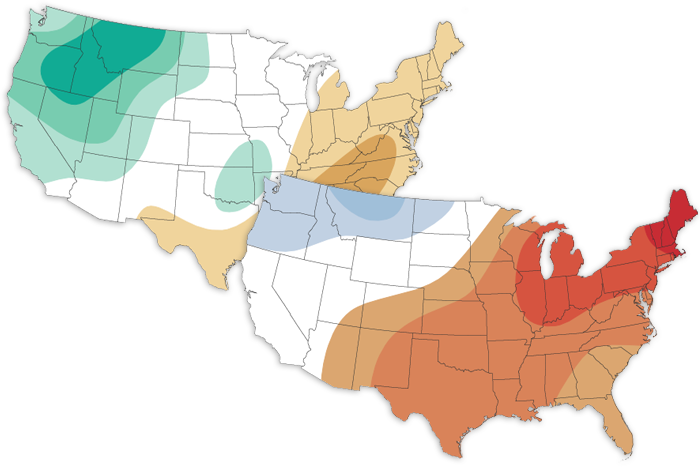
The November 2022 climate outlook favors a warmer- and drier-than-average month for the eastern United States and a cooler- and wetter-than-average month for the Northwest.
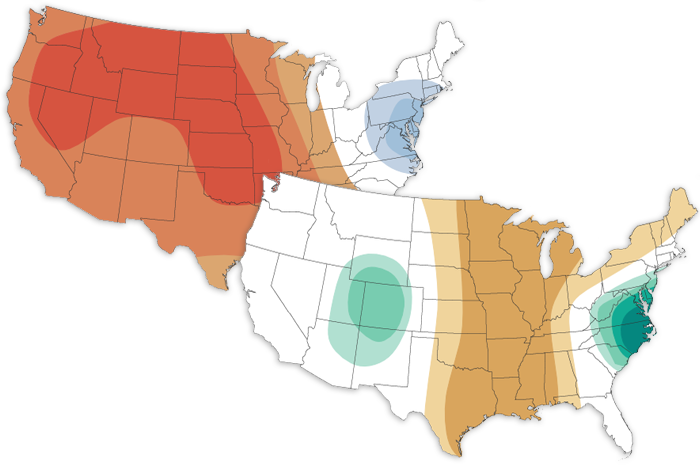
It's October. So in addition to talk of ghouls and goblins, we must chat about the October 2022 climate outlook. The outlook favors a hotter-than-average month for the western and central United States, and a colder- and wetter-than-average month for the Mid-Atlantic.

From the coasts of South America to the Galapagos Islands, the story of ENSO cannot be told without discussing its impact on marine life. But just as ENSO can affect climate patterns thousands of miles away from the equatorial Pacific, ENSO can also affect marine life. And there is no better example of this than its impacts on Salmon across the North Pacific. In this interview with expert Dr. Nate Mantua, learn all about the complexities of salmon and ENSO.
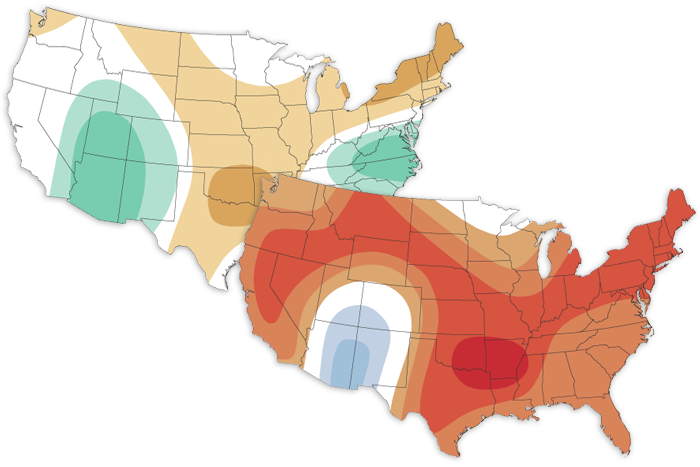
The August 2022 climate outlook favors a hotter-than-average month for much of the contiguous United States outside of the Southwest where the monsoon is expected to bring wetter and cooler-than-average conditions
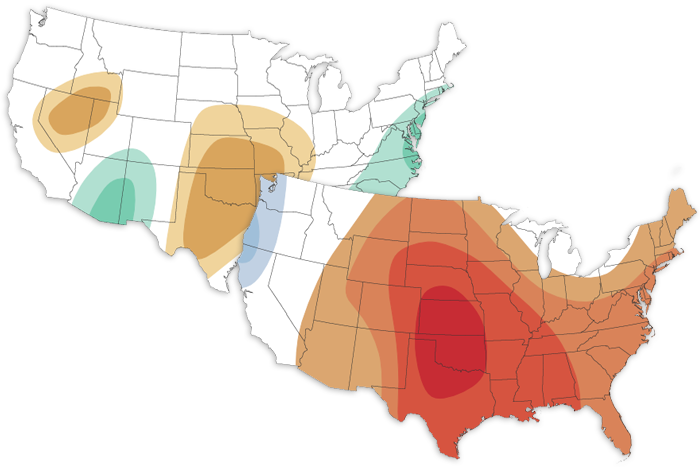
The July 2022 Climate outlook favors a hotter-than-average month for much of the country with a continuation of the wet start to the North American monsoon across the Southwest.
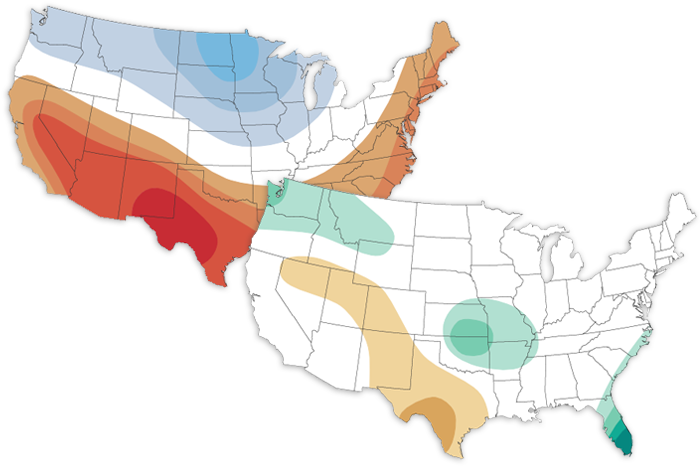
The June climate outlook favors a hotter-than-average start to summer for the southern and eastern United States and a cooler-than-average June for the north-central and northwestern U.S.
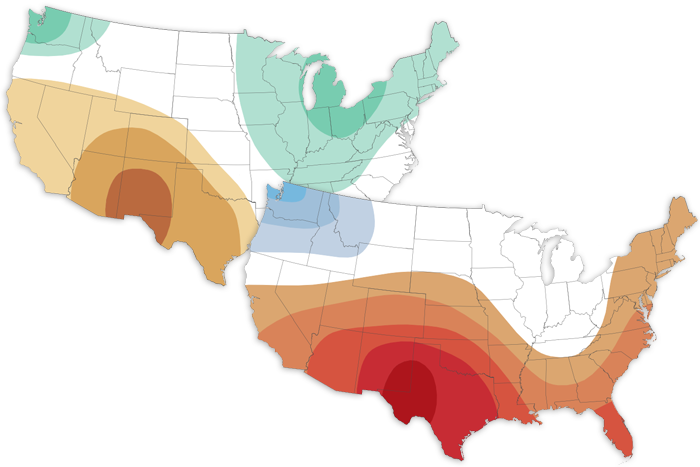
NOAA's April 2022 Climate Outlook favors a warm month across the southern and eastern United States, a dry month across the Southwest, and a wet month for the Great Lakes and Northeast.
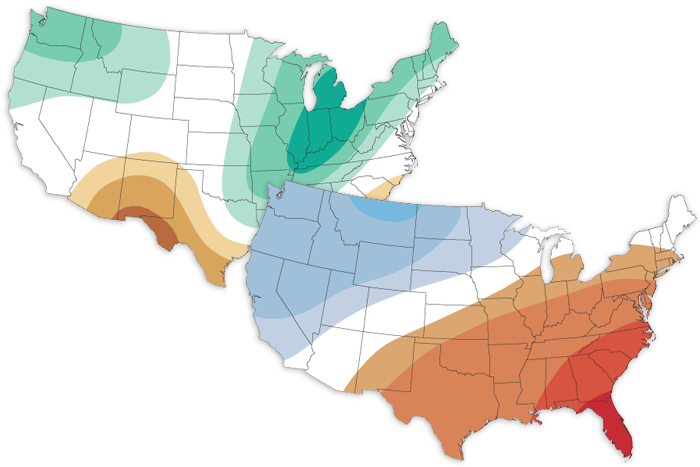
The March 2022 climate outlook favors a warmer-than-average month for the eastern/south-central US, a cooler-than-average month for the western/north-central US, and a wetter-than-average month for the Great Lakes.