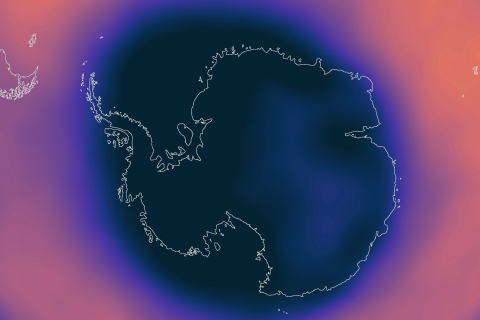
A strong polar vortex supported the formation of a large and deep Antarctic ozone hole in September 2020 that should persist into November, NOAA and NASA scientists reported today.

Climate.gov talks with Emily Fischer—an early-career atmospheric scientist and educator who has already made significant contributions to Earth science and fostering greater inclusion of women in the geosciences.

Weather balloons launched at the South Pole each spring routinely find areas of the Antarctic stratosphere where ozone has been completely destroyed. In 2019, they found no such areas.

The seasonal Antarctic ozone hole was the smallest on record in 2019, thanks to a warmer-than-average September.

If you missed our August 29 tweet chat, here's the transcript. Read what the fire and smoke experts had to say about the FIREX-AQ field campaign and its mission to study what's in the smoke from wildfires and agricultural burning.
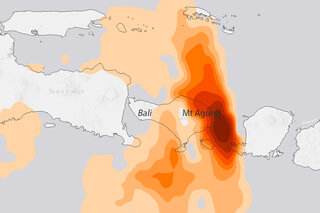
Mount Agung erupts in Indonesia: Is it a climate event?
December 7, 2017

The ozone hole didn't cause global warming, but climate and the ozone hole are related in other ways.

2013 continued a trend of water vapor in the surface atmosphere increasing over land and ocean relative to the 1970s, while the atmosphere over land becomes less saturated.

Like a prehistoric fly trapped in amber during dinosaurs' days, airborne relics of Earth's earlier climate can end up trapped in glacial ice for eons. How do climate scientists turn those tiny relics into a story about Earth's ancient climate?

From reindeer to regional temperature patterns, from sea ice age to Greenland surface melt, the Arctic Report Card is a yearly assessment of the Arctic's physical and biological systems and how they are changing. This collection of visual highlights from the 2013 report is a story of the Arctic in pictures.